
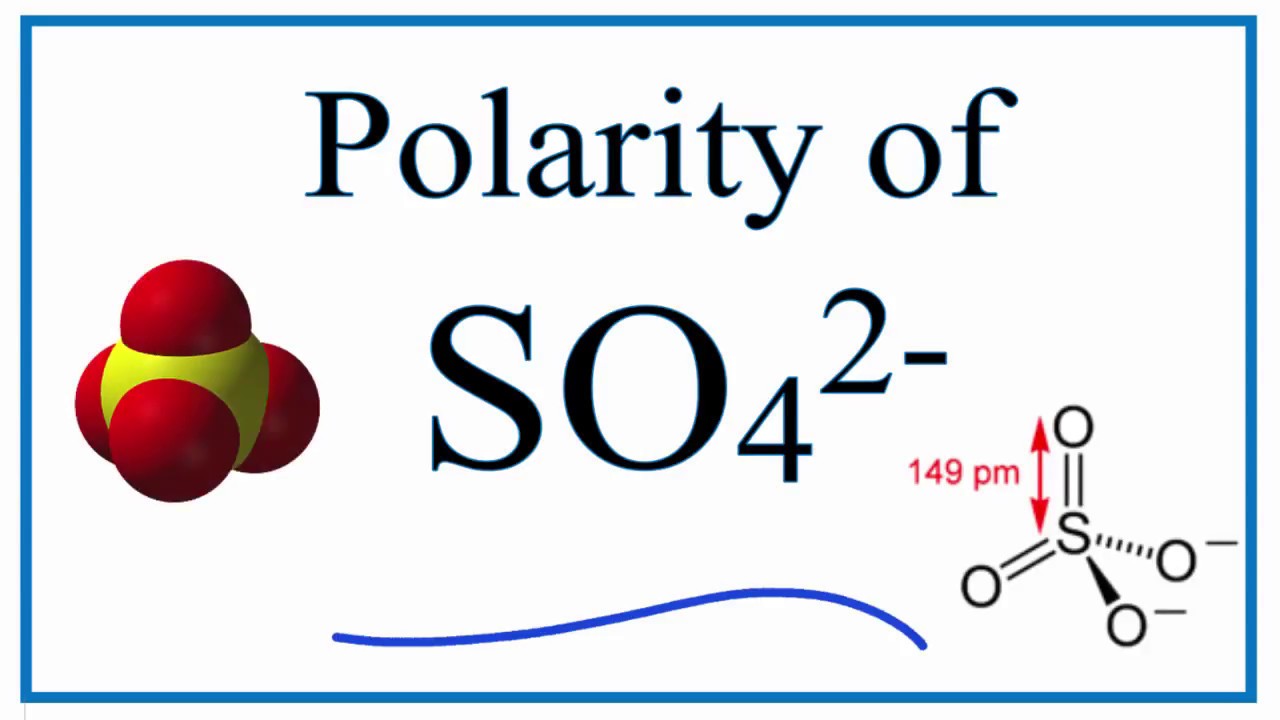
Structure 6: O₃ and O₁ form two double bonds with S, and O₂ and O₄ form a single bond with the S atom. There is a negative charge on both O₁ and O₄.

Structure 5: O₂ and O₃ form a double bond with S, and O₁ and O₄ form a single bond with the S atom. There is a negative charge on both O₁ and O₂. Structure 4: O₃ and O₄ form a double bond with S, and O₁ and O₂ form a single bond with the S atom. There is a negative charge on both O₃ and O₁. Structure 3: O₂ and O₄ form a double bond with S, and O₃ and O₁ form a single bond with the S atom. There is a negative charge on both O₃ and O₄. Structure 2: O₁ and O₂ form a double bond with S, and O₃ and O₄ form a single bond with the S atom. There is a negative charge on both O₂ and O₃. O₁ and O₄ form a double bond with S, and O₂ and O₃ form a single bond with the S atom. There are 6 resonance structures for sulfate ions which can be listed as follows. Let the oxygen atoms be numbered as O₁, O₂, O₃, O₄. Each single-bonded O carries a negative charge. Out of the four, two oxygen atoms form a double bond with S and the other two oxygen atoms form a single bond with S. Total valence electrons = (1 ✕ 6)S + ( 4 ✕ 6)O +( 2 ✕ 1)(e⁻)Įach structure contains sulfur as the central atom and the four oxygen atoms bonded to it. The sulfate ion has 32 valence electrons which contain 1 sulfur atom, 4 oxygen, and 2 negative charges.
#Sulfate ion bonding full
1.1 Functions and Continuity full solutions.1-6 Milestone - Pseudocode and Flowchart.Clemente Balderas Josue Eduardo M21S2AI3.
#Sulfate ion bonding pdf
Greek god program by alex eubank pdf free.1-2 Module One Activity Project topic exploration.Chapter 4 - Summary Give Me Liberty!: an American History.CH 13 - Summary Maternity and Pediatric Nursing.Chapter 15 Anxiety and Obsessive-Compulsive Disorders.General Chemistry I - Chapter 1 and 2 Notes.NY Times Paywall - Case Analysis with questions and their answers.Nclex HIGH Yield Official Quick Tip PDF.Business Core Capstone: An Integrated Application (D083).Comparative Programming Languages (CS 4402).Operating Systems 2 (proctored course) (CS 3307).Principles Of Environmental Science (ENV 100).Advanced Anatomy & Physiology for Health Professions (NUR 4904).
#Sulfate ion bonding professional
Professional Capstone Project (PSY-495).Professional Application in Service Learning I (LDR-461).Essentials for advanced professional nurse and professional roles (D025).Fundamentals of Biology: Cellular and Organ Physiology (BIO 203).Introduction to Computer Technology (BIT-200).Concepts Of The Nurse As Leader/Manager (NURS 4200).Adv Hlth Assess/Diagnostic Rea (NSG 510).Intro to Statistical Analysis (MAT-133).Art History I OR ART102 Art History II (ART101).Organic Chemistry Laboratory I (CHM2210L).Primary Concepts Of Adult Nursing (NUR 3180).Nursing Care of the Childbearing Family (NURS 125).Community Health and Population-Focused Nursing Field Experience (C229).Introduction to Biology w/Laboratory: Organismal & Evolutionary Biology (BIOL 2200).Health and Illness Across the Lifespan (NUR2214).



 0 kommentar(er)
0 kommentar(er)
